Unraveling Alzheimer's: New study documents how brain cells go bad
In the brains of people with Alzheimer's disease, there are abnormal deposits of amyloid beta protein and tau protein, and swarms of activated immune cells. But scientists do not fully understand how these three major factors combine to drive the disease. Now, UNC School of Medicine and National Institutes of Health researchers have untangled the mystery in lab experiments to reveal why one Alzheimer's drug currently in development shows promise and how other therapies might reverse the disease process.
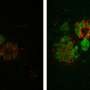
Led by Todd Cohen, PhD, assistant professor of neurology, UNC scientists used human cell cultures to show how amyloid beta can trigger a dramatic inflammatory response in immune cells and how that interaction damages neurons. Then they showed how that kind of neuron damage leads to the formation of bead-like structures filled with abnormal tau protein. Similar bead-like structures are known to form in the brain cells of people with Alzheimer's disease.
The UNC researchers also identified two proteins—MMP-9 and HDAC6—that help promote this harmful, amyloid-to-inflammation-to-tau cascade. These proteins and others associated with them could become drug targets to treat or prevent Alzheimer's.
"It's exciting that we were able to observe tau—the major Alzheimer's protein—inside these beaded structures," said Cohen, who is also a member of the UNC Neuroscience Center. "We think that preventing these structures from forming would leave people with healthier neurons that are more resistant to Alzheimer's."
The findings, published today in the journal Cell Reports, were made possible through a collaboration of three UNC labs led by Rick Meeker, PhD, Xian Chen, PhD, and Cohen, as well as the NIH lab of Jau-Shyong Hong, PhD.
To begin the study, Cohen and colleagues exposed immune cells normally found in an activated, inflammatory state in Alzheimer's brains to tiny clusters of amyloid beta—or oligomers, which are believed to be the most harmful forms of the protein.
"Our thinking was that the amyloid beta oligomers would activate an inflammatory response in these immune cells, as prior research suggested, and we wanted to see if this would induce pathological forms of tau when given to neurons," Cohen said.
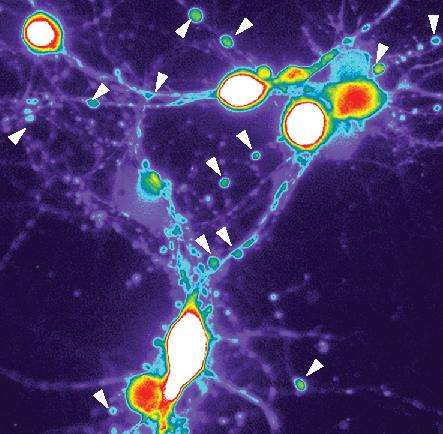
The researchers then focused on the fluid in which the immune cells had been growing. This fluid, which was filled with inflammatory factors—or proteins—resembled the fluid in which these cells typically live inside human brains. Cohen's team added this fluid to cultures of human cortical neurons. The neurons soon developed abnormal, bead-like swellings along their axons and dendrites.
This "neuritic beading" on axons and dendrites has been seen in Alzheimer's patients and has been considered an early sign of neuronal damage, although it hasn't been clear how beading was connected to abnormal tau or if the beading led to Alzheimer's disease.
Cohen's team then looked for tau in the beads and found a striking accumulation of it, though it was in an abnormal form and undetectable with the usual tools scientists use to detect the type of tau typically seen in Alzheimer's patients. Instead, the beaded tau was modified in a different way than previously thought. This modification is what Cohen said causes tau to become aggregated.
Tau proteins normally provide structural support for long, railway-like structures called microtubules, which are used to transport key molecules along axons. For reasons that have never been clear, tau proteins in Alzheimer's-affected neurons have a different pattern. They are detached from microtubules, bear abnormal chemical modifications, and clump into long, tangled, and thread-like aggregates. Whether these tau aggregates actively harm neurons isn't clear, but prior studies suggested that the loss of tau from microtubules and resulting disruption of axonal transport might cause serious damage.
The finding of abnormal tau in the neuritic beads indicated that these beads could mark tau's entry into the Alzheimer's disease process. Within the beads, Cohen's lab also found high calcium levels, which are known to harm neurons and are considered an important feature of neurons in people with Alzheimer's.
"We think these neuroinflammatory factors trigger this cascade," Cohen said. "They flood the neuron with calcium. And we think that once the calcium accumulates, it causes tau to become abnormally modified. This probably leads to a snowball effect: tau detaches from microtubules and is trafficked throughout the neuron, ending up in these beads. One possibility is that these tau-filled beads are the sites where the classic tangle-like aggregates of tau will eventually emerge, which is the hallmark of Alzheimer's disease."
A team led by collaborating researcher Xian Chen, PhD, associate professor of biochemistry and biophysics at UNC, used mass spectrometry to sort out the amyloid beta-induced neuroinflammatory molecules that had triggered the calcium influx and neuritic beading. They were able to show that one protein in particular, MMP-9, was responsible for some of this adverse effect.
"MMP-9 is an inflammatory protein shown to be elevated in the brains of Alzheimer's patients," Cohen said. "In our study, we show that MMP-9 alone can trigger a calcium influx that floods the neuron."
The researchers also identified the protein HDAC6, which originates from within neurons and concentrates in the neuritic beads. Normally, HDAC6 is thought to detect unwanted protein aggregates within neurons and transport them away for disposal. However, blocking HDAC6 stopped nearly all beads from forming in Cohen's lab experiments.
Both of these proteins have been found to be elevated in affected areas of Alzheimer's brains. Drug companies are now developing and testing HDAC6 inhibitors, which have performed surprisingly well in early studies, although it has not been fully understood how these inhibitors work.
"Our work might explain why HDAC6 inhibitors have shown such early promise," Cohen said. "And we think our work can help inform the development of other kinds of inhibitors that affect this cascade, particularly those that might impact cognitive processes."
A therapeutic strategy to block HDAC6—and/or MMP-9—might have applications beyond Alzheimer's. Neuritic beading is seen in several other neurodegenerative diseases as well as after head injury. Scientists have even observed beading to small extents in seemingly healthy elderly brains. Beading might be a general mechanism underlying cognitive decline, Cohen said.
In their study, Cohen and colleagues found some tau-filled neuritic beads in the brains of aged mice. And they discovered that chronic neuroinflammation could induce the beads to form in younger mice.
The researchers are now focused on creating a mouse model to confirm and further investigate the amyloid-to-inflammation-to tau process seen in this Cell Reports study.
"If we can demonstrate this cascade in a wild-type mouse, then we'll be able to study Alzheimer's and test therapies in ordinary lab mice without the need for artificial genetic engineering used in traditional Alzheimer's mouse models," Cohen said.