This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Pre-transplant microbiome sets the stage for graft-versus-host disease
Bone marrow transplants have saved the lives of thousands of blood cancer patients, but graft-versus-host disease, or GVHD, remains a debilitating and even life-threatening complication. A new study from Fred Hutchinson Cancer Center points to patients' pre-transplant gut microbiome as a major player in the development and severity of GVHD.
In the study, published in Immunity, the research team used animal models of GVHD and complex computational analyses to identify bacteria that help instigate GVHD, and others that suppress it. Microbiome data from blood cancer patients who received bone marrow transplants suggested some of the same bacterial groups may also influence GVHD in people.
"Many studies on GVHD and the microbiome focus on the microbiome after transplant," said Motoko Koyama, MD, Ph.D., a staff scientist in Fred Hutch's Hill Lab who led the study. "Our work shows that the pre-transplant microbiome could be the focus of therapies to reduce GVHD severity."
The work helps scientists better understand how GVHD develops and where to focus further translational studies aimed at preventing this often-devastating outcome. In particular the findings point toward approaches to modulate this pathway that can be tested clinically, said Geoff Hill, MD, who directs Hematopoietic Stem Cell Transplantation at Fred Hutch.
Untangling a deadly transplant complication
When blood cancers like leukemia strike, white blood cells that should fight infection have lost their way and begun growing out of control.
Bone marrow is the source of our immune cells, our oxygen-carrying red blood cells and our clot-forming platelets. To treat leukemia, doctors wipe out both cancerous immune cells and the bone marrow that can re-seed them, replacing them with healthy donated blood stem cells that will take root (engraft) and produce a new blood and immune system.
Donor immune cells do two things: They help replace cancerous immune cells, and they attack lingering leukemia cells in what's known as the graft-versus-leukemia, or GVL, effect.
Unfortunately, they sometimes also cause GVHD by attacking healthy recipient cells. Symptoms of this often-debilitating condition include vomiting, diarrhea, jaundice, skin rashes and heightened risk of infection. Severity ranges from mild to deadly. Acute GVHD arises within 100 days after transplant often occurs at regions where the body meets—and repels—the external world, like the gut and the skin. GVHD is the leading cause of non-relapse deaths in bone marrow transplant patients, and about 5,500 patients develop GVHD each year.
Scientists don't completely understand why this is so, but it's becoming clear that the microbes that call these areas home contribute to GVHD incidence and severity.
Koyama and Hill want to understand exactly how bacteria connect to the immune cells that do the damage. In a previous study, Koyama revealed that, unexpectedly, the epithelial cells lining the gut can give immune cells the helping hand they need to induce GVHD.
That work revealed the importance of an immune molecule called interleukin 12. The findings inspired a team led by Fred Hutch bone marrow transplant expert Stephanie Lee, MD, MPH, who holds the David and Patricia Giuliani/Oliver Press Endowed Chair in Cancer Research, to undertake a clinical trial testing an GVHD-preventive strategy aimed at inhibiting IL-12.
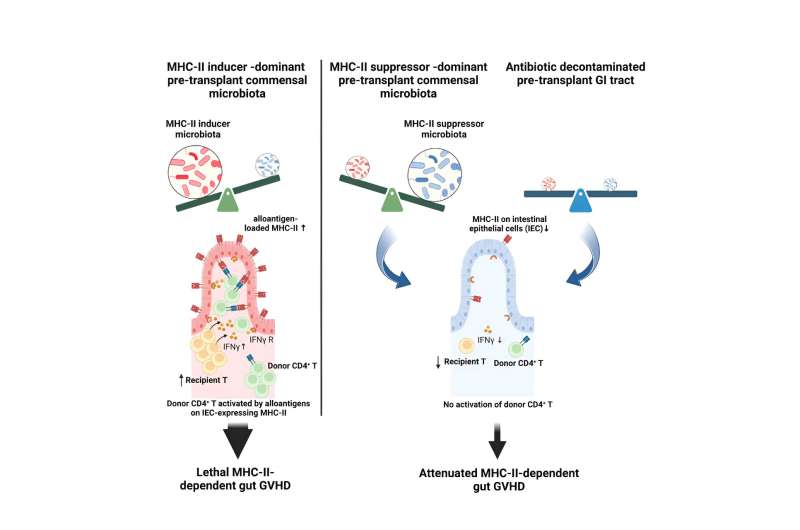
Koyama and the team also found that epithelial cells turned on a molecule called MHCII, or major histocompatibility complex class II, that allows them to communicate with immune cells. She saw that mice raised without a microbiome had little to no MHCII in their gut epithelial cells and also didn't get gut GVHD, suggesting that bacteria were playing a major role.
Bacteria can help or hinder GVHD
"We knew that the microbiome was important in causing GVHD via MHCII, but we didn't know which bacterial species were involved," Koyama said.
To help her uncover the bacterial players in GVHD, Koyama took advantage of the fact that the gut microbiome is shaped by environmental factors like food and water.
Over the long term, changing these factors changes the microbiome's makeup. The team surveyed genetically identical lab mice grown by different vendors and found that mice raised in different locations have different microbiomes and different levels of MHCII in their gut epithelium, and that mice with higher levels of gut MHCII get more severe GVHD.
To narrow down which bacterial species were involved in generating acute GVHD through MHCII, the researchers tackled the problem from both sides: by adding bacteria and taking them away.
First, Koyama and Hill teamed up with microbiome expert Sujatha Srinivasan, Ph.D., in Fred Hutch's Fredricks Lab. The collaborators used genetic sequencing to analyze the bacterial species present in mice from different vendors. Koyama collaborated with computational biologist Daniel Hippe, Ph.D., in Fred Hutch's Randolph Lab, to breakdown the correlations between bacterial groups and MHCII. They saw that certain bacterial genera—a taxonomic grouping broader than species—appeared to induce MHCII expression, while others suppressed it.
Then, Koyama put mice with different levels of gut MHCII in the same cages.
"This transfers the bacteria [between the mice] and we can see how this changes MHCII expression," Koyama said.
After four weeks, the MHCII-low mice had transformed into MHCII-high mice, and Koyama and Hippe saw that MHCII-inducing bacteria had moved into their gut microbiomes. She was also able to kill off "inducer" bacteria—and tamp down MHCII and GVHD—using certain antibiotics prior to transplant.
Unfortunately, Koyama also saw that treating mice with these antibiotics also reduced GVL, the ability of the donor cells to attack residual leukemia cells, although less severely than GVHD. In patients, GVL helps keep their cancers from recurring, so treatment strategies must balance the benefit of reducing GVHD with the risk of reducing GVL and enhancing recurrence.
"Another group had already shown that certain bacteria increase MHCII expression on gut epithelial cells," Koyama said. "We needed to know, do the suppressor bacteria have a functional effect?"
Working with Hutch colleague and microbiome expert Neelendu Dey, MD, the team grew two types of suppressor bacteria. Koyama pretreated two groups of MHCII-high mice with antibiotics to wipe out most of the "inducer" bacteria.
Two weeks of daily dosing with the suppressor bacteria ramped down their gut epithelial MHCII, while MHCII levels bounced back in mice that received antibiotics but no suppressor bacteria. Mice that received the suppressor bacteria after antibiotic treatment also survived better after transplant.
Koyama then collaborated with another Fred Hutch microbiome scientist, Kate Markey, Ph.D., MClin, to see if the human data supported her findings in mice.
They analyzed a cohort of microbiome data taken from bone marrow transplant patients prior to transplant at Memorial Sloan Kettering Cancer Center in New York City. While individual species differed a great deal between mice and human gut microbiomes, the researchers saw distinct overlap between GVHD-associated bacterial genera in mice and humans.
"This suggests that before transplant, humans also have different bacteria in their gut microbiota that can influence GVHD outcomes," Koyama said.
A window of opportunity
While the team's findings highlight the power of the microbiome in promoting GVHD, it will likely be some time before doctors will be able to modulate this complex ecosystem to improve transplant outcomes, she said.
But by further clarifying how interactions between the microbiome and donor and host immune cells precipitate GVHD, the researchers' work reveals the key interactions and players that can then become the focus of further research into therapeutic targets and new treatment strategies.
"There is still a lot to understand about how to manipulate the microbiome, but our work shows that the pre-transplant microbiome is an important place to focus future research," Koyama said.
More information: Motoko Koyama et al, Intestinal microbiota controls graft-versus-host disease independent of donor-host genetic disparity, Immunity (2023). DOI: 10.1016/j.immuni.2023.06.024