This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Exploring mechanisms of epigenetic regulation in fibrogenic activation of hepatic stellate cells in liver disease
Non-alcoholic fatty liver disease (NAFLD) has emerged as a leading cause of chronic liver disease worldwide, affecting approximately 38% of the global population from 2016 to 2019. NAFLD is characterized by the accumulation of fat in at least 5% of hepatocytes, and its severity ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), which can further progress to liver fibrosis (LF) and cirrhosis.
The complications of liver cirrhosis, primarily liver failure and portal hypertension, often result in unfavorable outcomes. Inflammation and cell death are key triggers of hepatic fibrogenesis in NAFLD, with hepatic stellate cells (HSCs) playing a crucial role in this process.
New insights on regulation of HSCs appear in a study published in the journal Gene Expression.
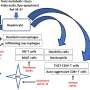
HSCs are central to the development of liver fibrosis in NAFLD. Under normal physiological conditions, HSCs remain quiescent and are involved in regulating retinoid homeostasis. However, various factors, such as lipid metabolites, free cholesterol buildup, and immune cell-associated pro-fibrotic molecules, can activate HSCs.
Once activated, HSCs undergo a process of transdifferentiation, acquiring profibrotic and proinflammatory properties, leading to excessive secretion of extracellular matrix proteins like type I and III collagen and fibronectin. This excessive deposition disrupts the normal liver architecture and function, contributing to the progression of liver disease.
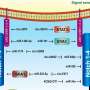
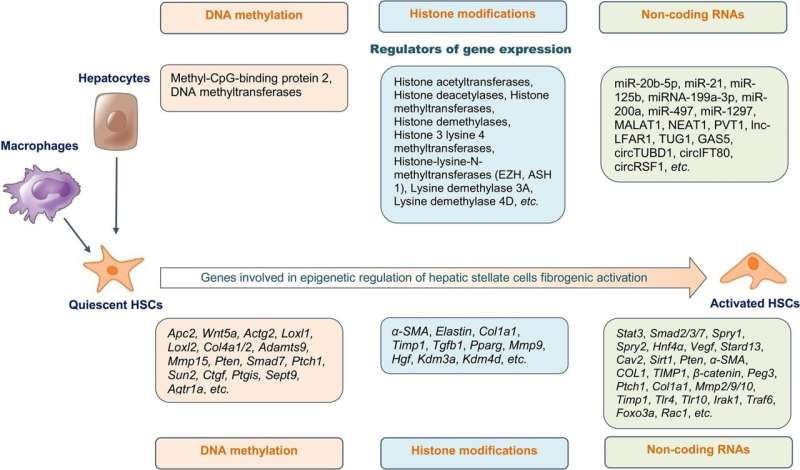
Epigenetic regulation is a key mechanism in the fibrogenic activation of HSCs, influencing gene activity without altering the DNA coding sequence. This regulation is stable and heritable, even after the causative factor is removed. Epigenetic modifications include DNA methylation, covalent histone modifications, chromatin remodeling, and non-coding RNAs (ncRNAs). These modifications can either enhance or repress gene expression, thereby playing a defining role in HSC activation and hepatic fibrogenesis.
DNA methylation, particularly at CpG-rich sites, is crucial in the transdifferentiation of HSCs. Research has shown that DNA methylation patterns are significantly altered during HSC activation. Hypomethylation and hypermethylation of specific genes have been associated with changes in gene expression, leading to HSC activation and liver fibrosis.
For instance, hypermethylation of the Ptch1 gene correlates with the maintenance of HSC activation, while methylation of the Ctgf gene promoter promotes hepatic fibrogenesis. This dynamic change in methylation status can serve as a potential biomarker for early detection and monitoring of disease progression.
Post-translational histone modifications, such as acetylation, methylation, phosphorylation, ubiquitination, and sumoylation, also play a significant role in regulating gene expression during HSC activation. These modifications influence chromatin structure, making DNA more or less accessible to transcription factors.
The presence of specific histone codes can either enhance or repress gene expression, thereby affecting the activation state of HSCs and contributing to liver fibrosis. Chromatin remodeling complexes, such as SWI/SNF and NuRD, further modulate the accessibility of transcriptional machinery to DNA, thereby fine-tuning the fibrogenic response.
NcRNAs, including microRNAs (miRNAs), long ncRNAs (lncRNAs), and circular RNAs (circRNAs), are involved in the epigenetic regulation of HSC activation. These ncRNAs can modulate gene expression at the transcriptional and post-transcriptional levels. For example, the lncRNA HOTAIR has been shown to promote HSC activation and liver fibrosis by modulating histone methylation and altering chromatin structure. miRNAs, such as miR-21 and miR-29, have been identified as key regulators of fibrogenic signaling pathways, making them attractive targets for therapeutic intervention.
The dynamic and reversible nature of epigenetic modifications presents promising prospects for developing targeted therapies for liver fibrosis in NAFLD. By targeting specific epigenetic mechanisms, it may be possible to reverse HSC activation and reduce fibrosis.
Molecular therapies aimed at correcting aberrant DNA methylation, histone modifications, and ncRNA expression are currently being explored as potential treatments for NAFLD-associated liver fibrosis. Small molecule inhibitors, antisense oligonucleotides, and CRISPR-based gene editing tools are among the strategies being developed to modulate the epigenetic landscape in fibrotic livers.
NAFLD is a prevalent liver disease with significant morbidity and mortality due to its progression to liver fibrosis and cirrhosis. HSCs play a pivotal role in hepatic fibrogenesis, and their activation is tightly regulated by epigenetic mechanisms. Understanding the epigenetic regulation of HSC activation provides valuable insights into the pathogenesis of NAFLD and offers potential therapeutic targets for reversing liver fibrosis.
Continued research in this field is essential for developing effective treatments to mitigate the impact of NAFLD on global health. This approach holds promise for transforming the management of liver fibrosis, potentially improving outcomes for millions of individuals affected by NAFLD.
More information: Dmitry Victorovich Garbuzenko, Mechanisms of Epigenetic Regulation in the Fibrogenic Activation of Hepatic Stellate Cells in Non-alcoholic Fatty Liver Disease, Gene Expression (2024). DOI: 10.14218/GE.2023.00090