Savior of T-cells may be enemy of liver immune cells
Researchers at Houston Methodist demonstrated that a surface protein called OX40, responsible for keeping one type of immune system cell alive, can trigger the death of liver immune cells, in turn starting a chain reaction of events leading to liver inflammation and disease.
The study, led by Xian Chang Li, M.D., Ph.D., is described in the April 24 online issue of the Journal of Clinical Investigation (JCI).
OX40 is a cell surface receptor on T-cells, the white blood cells (leukocytes) that circulate in our bodies. The liver hosts a large number of immune cells, and this protein receptor helps T-cells proliferate. Outside the liver, OX40 drives cell longevity, preventing emergence of proteins that threaten T-cell survival.
However, Li's team observed in a genetically modified mouse that this same OX40 protein killed immune cells inside the liver in what is called pyroptotic death, or inflammatory cell death.
"What we found was polar opposite of what our team originally hypothesized. Invariant natural killer T-cells, or iNKTs, are immune cells in the liver that express OX40 on their surface," said Li, director of the Immunobiology and Transplant Science Center at Houston Methodist Research Institute. "Instead of helping these resident immune cells in the liver to survive, OX40 drives a messy inflammatory cell death."
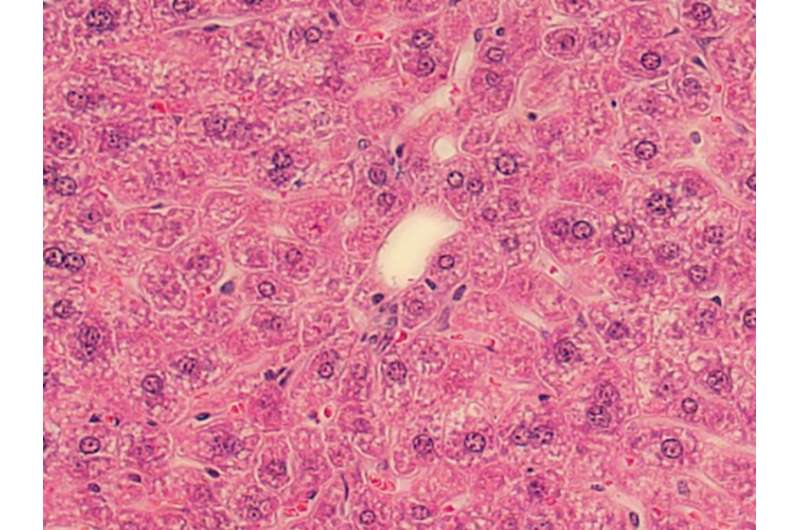
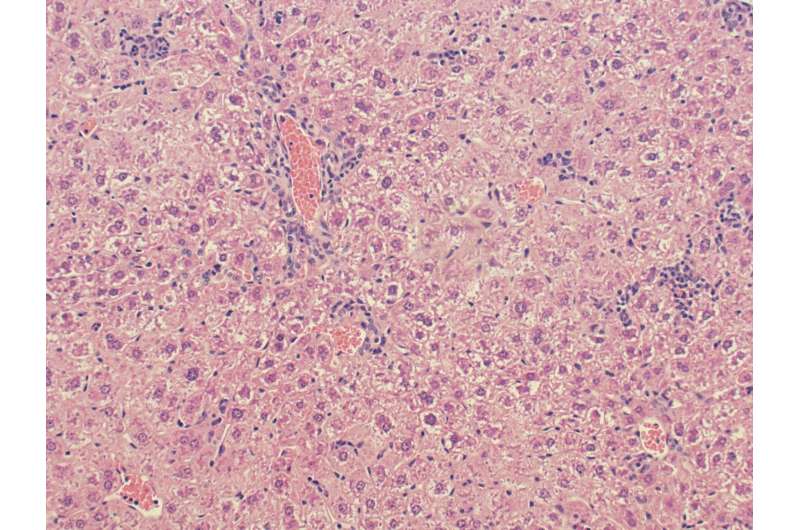
Li theorizes that OX40's threat to the liver is a two-step process. First, OX40 activates a chain reaction with the production of another protein (caspase-1), which, in turn, activates a third protein (gasdermin D) that forms pores in cell membranes. Once the membranes become permeable to circulating molecules, the immune cells commit suicide by bursting, releasing the inflammatory messengers of the immune system (cytokines) and causing inflammation.
Second, the OX40-induced death of the resident immune cells (iNKTs) is what likely leads to the serious consequences for liver inflammation, liver cell damage and, in severe cases, cirrhosis.
"We are clearly interested in learning how the liver microenvironment makes the iNKT cells so fragile and susceptible to this messy cell death," Li said. "We hope to open new avenues of intervention such as OX40 inhibitors or blockers."
Once a blocker is designed to stop inflammatory cell death, Li and his colleagues plan to run clinical studies in humans to see if the same events take place. According to the American Liver Foundation, 30 million Americans suffer from some form of liver disease.
More information: Peixiang Lan et al, TNF superfamily receptor OX40 triggers invariant NKT cell pyroptosis and liver injury, Journal of Clinical Investigation (2017). DOI: 10.1172/JCI91075